仹734-0036 峀搰導峀搰巗撿嬫夃侾亅俀亅3
TEL. 082-257-5142
What is constitutive activated G protein-coupled receptors?
Among the various receptors expressed on cell membranes, G protein-coupled receptors (GPRs) constitute the largest family of receptors, and the existence of approximately 800 types of GPRs in humans has been demonstrated, making them an important target for drug discovery.
GPR3 is a G protein-coupled receptor that was first cloned from rat brain by Yoshishu Saeki of Osaka University (currently Director of Saeki Hospital) in 1993. Subsequently, it was reported that GPR3 and its family receptors, GPR6 and GPR12, have the unique ability to activate homeostatic Gs and to raise and maintain intracellular cAMP.
Since the discovery of the involvement of oocyte-expressed GPR3 in meiotic arrest in collaboration with Mehlmann, Jaffe, Saeki and colleagues (Mehlmann et al., Science 2004), Dr. Tanaka, the principal investigator, has been studying the functions and roles of GPR3, mainly in the central nervous system. We have been studying the functions and roles of GPR3 mainly in the central nervous system. As a result, we have shown that GPR3 is mainly expressed in neurons in the CNS and is deeply involved in neuronal homeostasis, including projection elongation, differentiation, and survival, and that its disruption exacerbates pathological conditions such as cerebral infarction. Recently, GPR3 has also been found to contribute to axonal regeneration and influence glaucoma pathology.
GPR3 is expressed not only in neurons but also in immune-related cells and contributes to the regulation of nuclear receptor NR4A2 expression and to the suppression of effector T cells (Shiraki et al., JPS 2022). The role of GPR3 in the pathogenesis of multiple sclerosis is currently under investigation.
Other researchers have reported a link to Alzheimer's disease (Thathiah A et al., Science 2009; Huang Y et al., Sci Transl Med 2015) and homeostasis, including energy metabolism in brown fat cells (Johansen OS et al. (Johansen OS et al., Cell 2021), and it is a receptor that has attracted considerable attention in recent years.
Principal investigator Tanaka believes that in natural life forms, diverse proteins, receptors, and gene expression are mutually involved in maintaining homeostasis of the organism, and that no single molecule is useless.
By exploring the common functions and molecular basis of neuro-immunity through homeostatically activated receptors, he aims to understand the pathology of neuroimmune diseases such as cerebral infarction and multiple sclerosis, and to apply his findings to the treatment of these diseases.
1. GPR3 promotes neurite outgrowth and neuronal polarity formation
丂The adult mammalian central nervous system does not recover from neurological damage because myelin inhibition prevents axonal regeneration after CNS injury, and GPR3 and its family receptors, GPR6 and GPR12, have been shown to exhibit myelin-resistant neurite outgrowth (Tanaka et al., JBC. 2007). Furthermore, GPR3 is transported to neurite tips and contributes to PKA activation at the apical region (Miyagi et al., PLoS One 2016). More recently, we have also shown the detailed expression distribution of GPR3 in the central nervous system (Ikawa et al., Brain Res 2021) and that GPR3 contributes to neuronal polarity formation during neuronal differentiation (Tanaka et al., MCN 2022).
2. GPR3 modulates cerebellar granule neuron differentiation
We have shown that GPR3 is upregulated in the intracerebellar granule neuron layer during postnatal cerebellar development and is involved in neuronal differentiation and maturation (Tanaka et al., PLoS One. 2009).
3. GPR3 contributes to neuronal survival under ischemic conditions
GPR3 is important for cell survival and homeostasis, and has been shown
to act in a cytoprotective manner against ischemia-related stresses such
as hypoxia and ROS stress; GPR3 knockout mice show enlarged infarct foci
compared to wild-type mice (Figure 1) and are vulnerable to ischemic stress
( Tanaka et al., Neurobiol Dis 2014).

GPR3 knockout mice have enlarged cerebral infarct foci
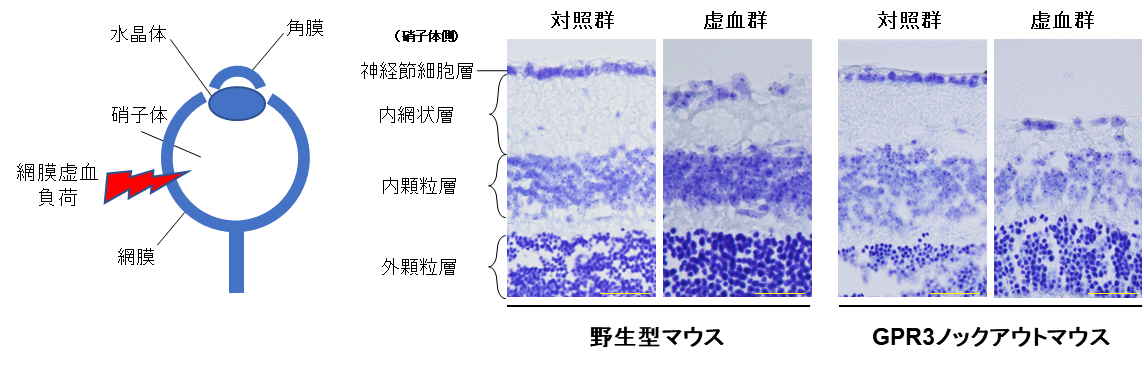
4. GPR3 is expressed in retinal ganglion cells and contributes to cell survival and axon regeneration
GPR3 is relatively abundantly expressed in retinal ganglion cells in the mouse retina and is involved in suppressing neuronal cell death in response to aging and ischemic stress. Furthermore, GPR3 gene transfer to retinal ganglion cells contributes to axonal regeneration after optic nerve injury (Masuda et al., Neurobiol Dis 2022)
 丅
丅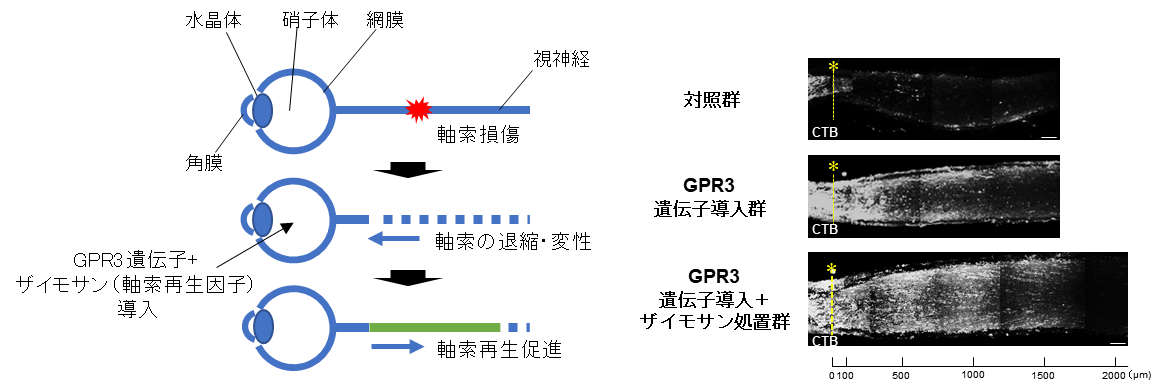
Main Publications (*Responsible Author)
1.
2. .
Potential role of inducible GPR3 expression under stimulated T cell conditions.
J Pharmacol Sci. 2022 Mar;148(3):307-314. doi: 10.1016/j.jphs.2022.01.005.
3.
GPR3 accelerates neurite outgrowth and neuronal polarity formation via
PI3 kinase-mediating signaling pathway in cultured primary neurons.
Mol Cell Neurosci. 2022 Jan;118:103691. doi: 10.1016/j.mcn.2021.103691.
4. Ikawa, F., Tanaka S*., Harada, K. Hide, I., Maruyama, H., and Sakai, N.
Detailed
neuronal distribution of GPR3 and its co-expression with EF-hand
calcium-binding proteins in the mouse central nervous system.
Brain Res. 2021 Jan 1;1750:147166. doi: 10.1016/j.brainres.2020.147166.
Epub 2020 Oct 16
5. Miyagi T, Tanaka S*, Hide I, Shirafuji T, Sakai N The Subcellular Dynamics of the Gs-Linked
Receptor GPR3 Contribute to the Local Activation of PKA in Cerebellar Granular
Neurons. PLoS One. 2016 Jan 22;11(1):e0147466. doi: 10.1371/journal.pone.0147466. eCollection
2016. [PubMed]
6. Tanaka S.*, Miyagi, T., Dohi, E., Seki, T., Hide, I., Sotomaru, Y., Saeki, .Y,
Antonio Chiocca, E., Matsumoto, M., Sakai, N. Developmental expression
of GPR3 in rodent cerebellar granule neurons is associated with cell survival
and protects neurons from various apoptotic stimuli. Neurobiol Dis, 68C: 215-227, 2014 [PubMed]
7. Tanaka S, Shaikh IM, Chiocca EA* and Saeki Y, The Gs-Linked receptor GPR3 inhibits
the proliferation of cerebellar granule cells during postnatal development,
PLoS One, 15: e5922, 2009.
8. Tanaka S, Ishii K, Kasai K, Yoon SO and Saeki Y*: Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 upregulates cyclic AMP levels and promotes neurite outgrowth. J Biol Chem, 282: 10506-10515, 2007. [PubMed]
9. Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM and Jaffe LA*
Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive
activator of the Gs G protein. J Cell Biol, 171: 255-265, 2005.[PubMed]
10. Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ and Jaffe LA*
The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes.
Science, 306: 1947-1950, 2004.